Every vehicle has a #battery that used for starting engine and powering electrical devices .
The lead acid battery consists of an insulator container contains tow poles off lead and acid electrolyte
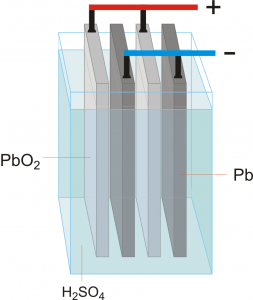
When we put lead in #sulphuric_acid electrolyte it reacts and converts lead poles into #Lead sulphate PbSO4 .
Charging : When connecting electrodes to DC the anode converts into PbO2 and the cathode converts into Pb
Discharging : When connecting electrodes to load they converts into PbSO4

The scheme below shows the detailed chemical electrode reactions:
| Discharge | Charge | ||
|---|---|---|---|
| Positive Electrode Anode (+) |
Negative Electrode Cathode (-) |
Positive Electrode Anode (+) |
Negative Electrode Cathode (-) |
PbO2 + HSO4– + 3H+ + 2e– PbSO4 + 2H2O |
Pb + HSO4– PbSO4 + H+ + 2e– |
PbSO4 + 2H2O PbO2 + HSO4– + 3H+ + 2e– |
PbSO4 + H+ + 2e– Pb + HSO4– |
| Overall Cell Reaction | Overall Cell Reaction | ||
Pb + PbO2 + 2H+ + 2HSO4– 2PbSO4 + 2H2O |
2PbSO4 + 2H2O  Pb + PbO2 + 2H+ + 2HSO4– |
||