A filter that converts these gases to less toxic gases it called catalytic converter
In the ideal case, the #combustion inside vehicles #engine results a group of gases consisting of #carbon dioxide and #water vapor, but in the normal case, other gases such as carbon monoxide resulting from incomplete combustion (hydrocarbons) also result from incomplete combustion and Nitrogen oxides (NOx) resulting from high temperature inside the combustion chamber are produced.
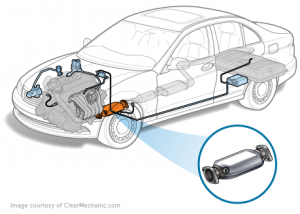
These gases are highly toxic to the environment in general, so vehicles developers are trying to reduce the amount of these gases as much as possible by adding a device at exhaust line in a cylinder containing a filter that converts these gases to less toxic gases it called catalytic converter
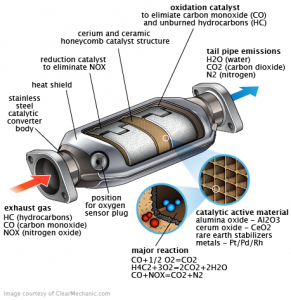
This filter is made of ceramic or silica and aluminum oxide. Silicon oxide in a shape of a lot of a longitudinal tubes with a large reaction surface saturated with catalysts such as Platinum , Rhodium and Palladium they are a precious metals

. Each filter contains about 1-2 grams of Platinum in small cars and up to 12 grams of platinum in large trucks .
Rhodium acts as a reducer to nitrogen oxides and palladium acts as an oxidizer for carbon monoxide. Platinum acts as oxidizer and reducer at the same time
The Chemical reactions :
Nitrogen reduction :
2CO + 2 NO → 2 CO2 + N2
hydrocarbon + NO → CO2 + H2O + N2
2H2 + 2 NO → 2 H2O + N2
Oxidation of carbon monoxide and hydrocarbons :
2CO + O2 → 2 CO2
hydrocarbon + O2 → H2O + CO2




